R&D Center in China
Key R&D Capabilities
Long-acting and Extended Release Technology
Liposome and Targeted Drug Delivery Technology
New Molecular Entity Platforms
Biological Antibody Technology
Innovative Medical Technology
R&D Center in the U.S.
Key R&D Capabilities
International R&D Collaboration
Exploratory Study for Innovative Drugs
R&D Center in Europe
Key R&D Capabilities
Transdermal Drug Delivery Technology
Granted Patents
272 patents granted in China
586 patents granted overseas
(As of June 2025)

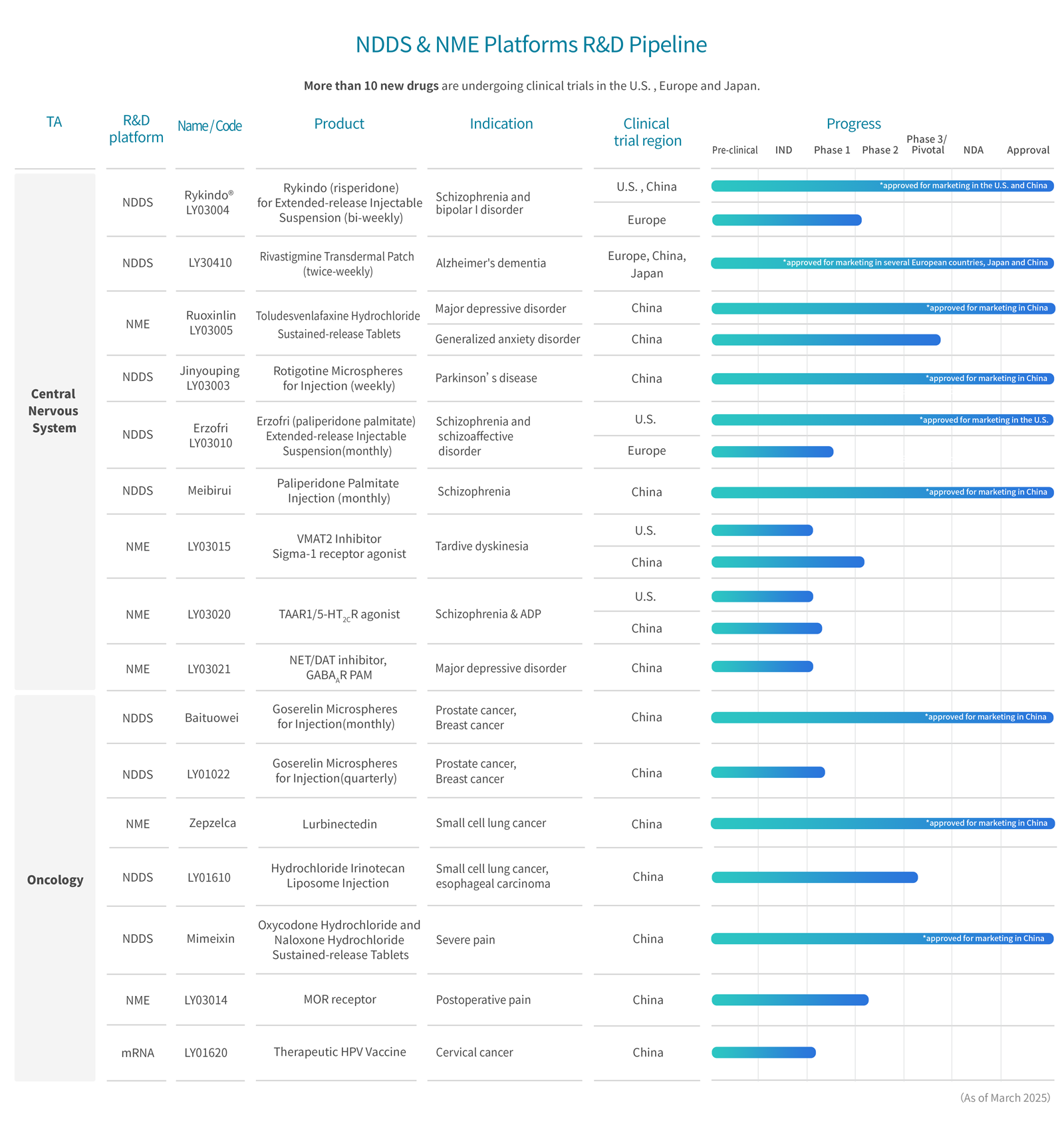
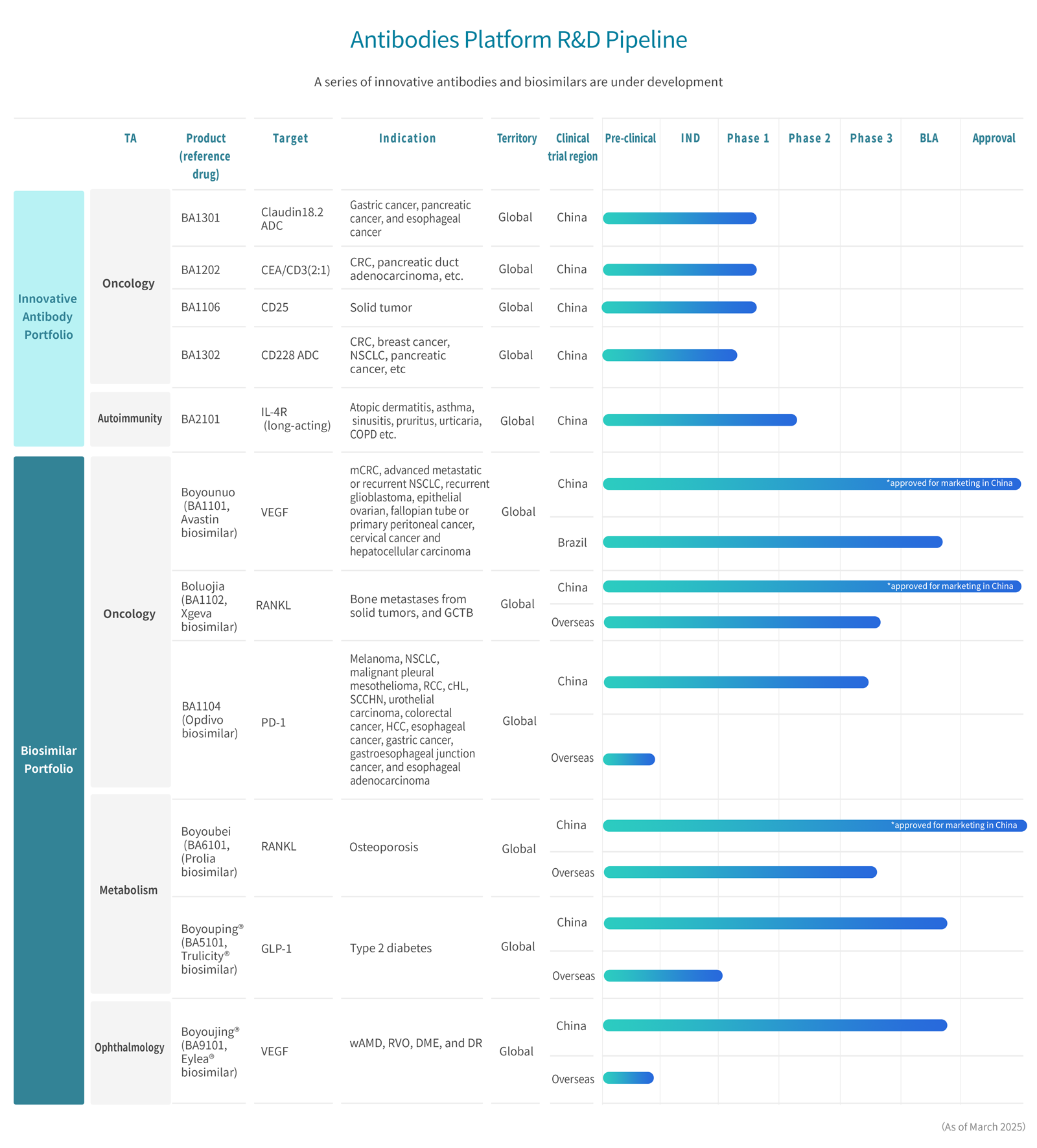
R&D Pipelines